2015/01/21 from Climate of Sophistry
What is Insulation, And what Does it Do?
People (well, the climate alarmists) don’t seem to understand what “insulation” is. They think that it means that it makes heat “pile up” inside the source of heat, or in the medium between the insulation and source of heat, so that the source of heat and/or the medium will get hotter than the source of heat and power input.
There is no such thing as “heat pile up”. This is a non-existent concept. You can think of it, like you can think of a unicorn, but it doesn’t exist. Heat does not pile up, it readily and freely flows into whatever is around it.
Insulation is something that only works in a gaseous environment – it is all about a gaseous environment. Insulation, a blanket, a greenhouse, all work the same way, and that way is preventing convective cooling and air circulation. Insulation in the form of a blanket, a sweater, a greenhouse enclosure, home insulation, etc., is about reducing and eliminating convective cooling, i.e. the loss of warm air. A blanket, or insulation, etc., is about doing the opposite of what the atmosphere does!
In your house, insulation helps prevent the furnace-heated air from escaping your house and being replaced with cold air from outside. It doesn’t make the furnace burn hotter. In your water heater, it helps the water retain its temperature after it has been heated. It doesn’t make the water hotter than the heater.
You can wrap a heat source with as much insulation as you want. All that will happen is that the insulation will reach the temperature of the heat source, and the heat source will not rise in temperature. Insulation is just matter, just material like anything else. When exposed to heat, it will warm, and will conduct that heat outward via diffusion.
Free Energy
People have claimed that if you have a heating element inside a mug of coffee, that if you then wrap the mug in insulation, the coffee and heating element will get hotter and hotter and hotter, because of “heat pile up”.
Rather, the insulation would simply help keep the mug from cooling once the power is removed from the heating element, otherwise, the insulation will simply attain the temperature of the heating element, if left long enough.
Imagine if we could heat coffee, i.e. water, this way? You just wrap enough insulation, and then a heating element at 60C inside the water can cause the water to boil because of “heat pile up” in the water due to the insulation around it!
This violates all of thermodynamics. We’ve been trying to do stuff like that for hundreds of years. The discovery of the laws of thermodynamics are the result of those attempts.
Same with the steel greenhouse. The claim is that if you keep on adding shells, the inner sphere will get hotter and hotter by a multiple of the number of shells.
If the steel greenhouse worked that way, then you could power a steam engine and get more work out than you put it. You could layer a few shells around the inner source, make the inner source multiple times hotter than the tiny input at the centre, then flood it with water and have instant explosive steam generation. Then repeat over and over. They found back in the 1700’s and 1800’s that reality didn’t work this way.
Diffusive & Radiative Transfer are not Opposite Thermodynamics
If you add a new layer of steel physically directly touching an internally heated sphere, this new layer will simply heat to the temperature supplied from the interior sphere. In fact, the new layer will be a little cooler because it will have a larger surface area than the original sphere.
The interior won’t get hotter because it heats a new layer of steel on top of it. In this case you have the diffusion transfer equation, which similarly has a differential of hot and cold terms describing the heat flow, as does the radiation transfer equation, and we all understand that heat does not physically diffuse from cold to hot and that physical contact between a cold object and warm object does not make the warmer object warmer still.
Visually, think of it as in the following diagrams (read the captions please):
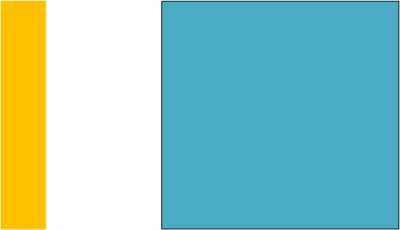
Initial configuration. There is a heated element (left, yellow rectangle) with constant power input thus holding a constant temperature. There is also a passive, unheated, cold object being brought in (right, blue square).
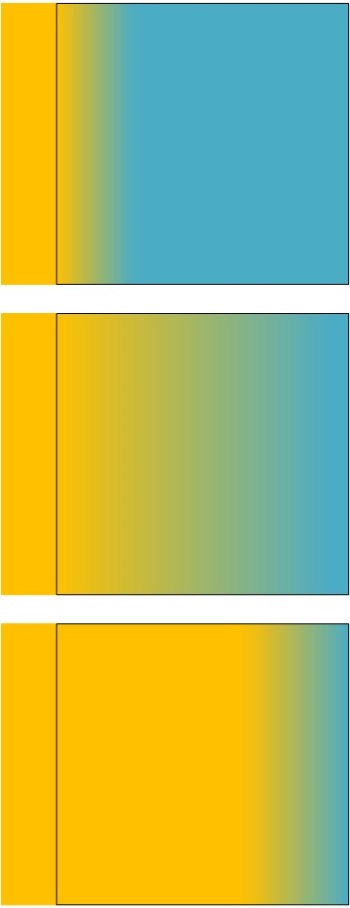
The passive block is brought into physical contact with the powered heat source. Heat begins transferring via diffusive (conduction) heat transfer from the powered source (rectangle) to the passive block (square) and raises the temperature of the passive block. At 3 sequential times, the temperature of the passive block is equilibrating to equal the temperature of the heat source. Neither the block nor the heat source can become hotter than the original temperature of the heat source, since there is no additional power being supplied to the heat source.
Putting a vacuum gap between the objects does not invert the laws of thermodynamics!!
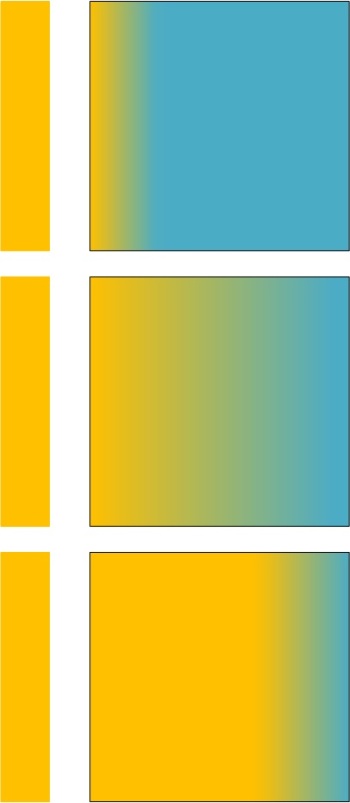
With a vacuum gap in between the source of heat and the passive block, radiation transfers heat from the heat source to the passive block and the block rises in temperature to that of the heat source. The heat energy then continues conducting through the block to its other side: this is how and why energy is conserved without the passive block “back-heating” the source.
Insulation, the passive block, does not cause “heat pile up”. Heat transfers one way only, and cold does not lead to hot becoming hotter still because it is being heated up.
The atmosphere is not a blanket. The atmosphere does not have heat piling up inside it. And we’re not wrapping the atmosphere in insulation. And adding “insulating cream” to coffee does not make the coffee have a higher final temperature than what it is heated with, etc.
There is no radiative climate science greenhouse effect. The entire field of climate science is a scandal which exploits the lack of knowledge of the Laws of Thermodynamics in the general populace, and even in the scientific community itself. Climate science, as based on its radiative greenhouse effect and its “heat pile up” postulate, is founded on an entirely irrational and non-existent premise, as we see the result of for example in the last post. The foundation of its ontology is wrong, the foundation of its physics is non-existent, thus, none of its alarmist claims which are directly based on that false ontology can be correct. The alarmism is directly dependent upon that false ontology. Hence, alarmism is false, along with much of the rest of the field of climate science itself.
Climate Pseudoscience Thermodynamics
The following diagram is how climate science thinks of heat flow and thermodynamics, and all others who subscribe to “steel greenhouse” ideas and the climate science radiative greenhouse effect:
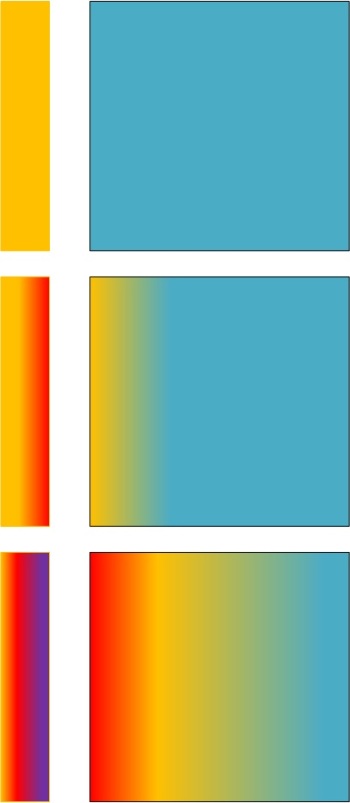
Top: A passive cold object (blue, square) is brought near a heat source (rectangle, yellow). Middle: As the passive cold object is warmed by the heat source, it presents back-resistance to heat flow from the source of heat. Bottom: This requires the source of heat to heat up some more so that it can continue sending heat energy to the passive object.
There’s no end to this process – it results in a constant runaway mutual heating that never ends. Think of that: mutual heating! There is no mutual heating. Heat is only one way, fellas.
Don’t you think we would have noticed that all we needed to do to make a source of heat hotter still was to simply to bring something cold nearby? Well, we’ve tried to notice that, we’ve spent hundreds of years trying to notice that. The discovery of the Laws of Thermodynamics have been the result. There is no such thing as back-resistance to heat flow.
We have the mathematics of heat flow. We know how it works, we know why it works. It has been known for around 200 years. The differential equations of heat flow and conservation of energy simply do not do what is claimed by the climate science radiative greenhouse effect. Look at the diagrams above, and think about it.
No comments:
Post a Comment